A sticky matter
Biofilm and catheter-related infections
It’s a dilemma that has kept scientists busy for decades: Central-venous catheters can be life-saving, but also come with life-threatening risks. Catheter-related bloodstream infections (CRBSI) continue to rank among the most common causes for rehospitalisations and even deaths in dialysis and parenteral nutrition.
Undoubtedly, CRBSI must be avoided at all costs – but experts have yet to agree on the best method to do so. In 2022, Austin et al. again pointed to the persisting “uncertainty about ‘prophylactic’ agent selection”. (1) While researchers are still exploring different avenues, one variable remains at the forefront: a significant correlation between infections and biofilm. This article takes a closer look at this phenomenon – from the biological mechanisms behind it to its implications for effective catheter management.
What is biofilm?
When scientists use the term “biofilm”, they generally refer to a thin layer of microorganisms which develops on various wet surfaces. In central-venous catheters (CVC), those layers mainly consist of:
- bacteria, fungi, and the matrix produced by these organisms
- blood material and fibrinous sheath
- minerals and lipids
- administered drugs
An accumulation of those substances will sooner or later affect the catheter’s functionality. On one hand, biofilm impedes the blood flow and thereby results in a lower patency rate. On the other, it provides a perfect breeding ground for all sorts of infectuous germs.

How does biofilm lead to infections?
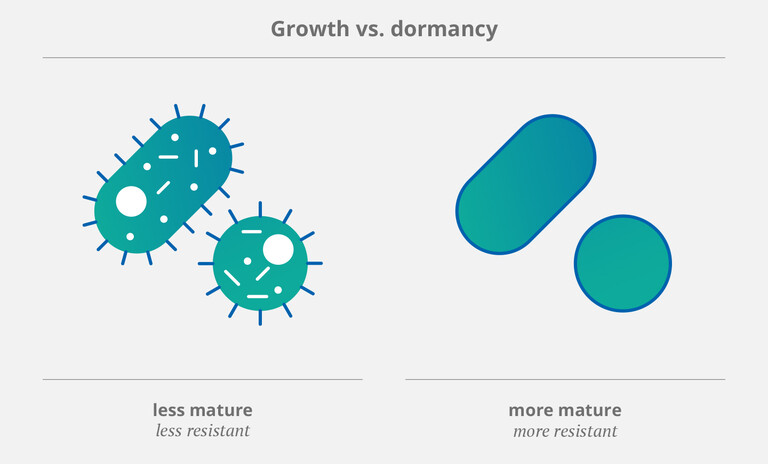
A study published in 2022 identified two types of bacteria as “common, if not the most common causes” of CRBSI: gram-negative Escherichia coli (E. coli) and gram-positive Staphylococcus epidermidis (S. epidermis) (1). Biofilm not only helps those microbes find their way into the catheter – it also protects them against the body’s own defense mechanisms and antimicrobial agents. Simply put: The more biofilm gathers within a catheter, the easier it becomes for germs to accumulate and spread diseases.
It’s important to note that microorganisms (such as bacteria and fungi) employ specific strategies to survive in adverse environments. One of these is to stop growing and enter a state called “dormancy”. (2) This allows pathogens to tolerate attacks from the immune system as well as exposure to antiobiotics. Therefore, dormant or “mature” (and thus more resistant) bacteria (3, 4) pose a particular threat to patients with CVCs. As Rittershaus et al. pointed out in a 2013 review, they occur “in relatively high numbers in bacterial biofilms”. (2)
The problem with antibiotics
What makes biofilm so hard to combat is the fact that it comprises so many different microorganisms. Scientists have been struggling to find a solution that proves effective against all of them: While one treatment method might kill some bacteria, others will still remain within the catheter – especially those that have entered a “dormant” state. Traditionally, doctors have been prescribing antibiotics to manage CRBSI. But this treatment has proven insufficient for several reasons:
Antibiotics only kill a limited number of germs.
Antibiotics lead to bacterial resistances in the human body – which means they will likely become ineffective over time.
Antibiotics can have other unwanted effects. Therefore, their use should be limited whenever possible. (5)
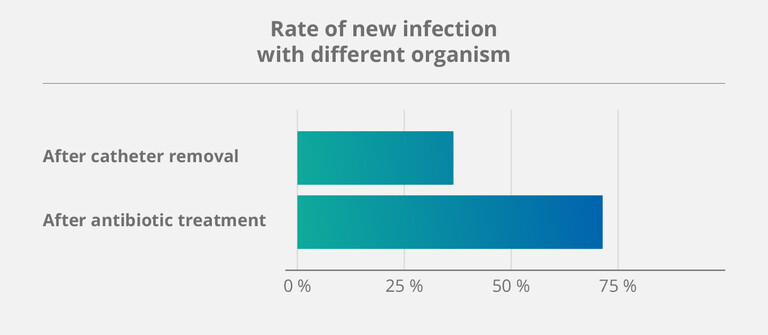
The importance of preventive – rather than reactive – measures was once again confirmed by a study on salvage strategies for CVCs, published in 2023 (6). Over a five-year period, more than 140 patients dependent on home parenteral nutrition (HPN) underwent different treatment methods. This led to the following findings:
- Treating already infected catheters with antibiotics had a high success rate.
- But: Catheters salvaged in this manner also had a 71 % rate of developing another infection afterwards.
According to Taleb et al., a possible explanation for this phenomenon might be exactly those dormant germs – because they keep lingering within the “sticky” parts of the biofilm that antibiotics cannot fully destroy. (6)
The antimicrobial key to effective catheter locks
As the old saying goes, prevention is always better than cure. This holds especially true when it comes to CVCs that protect – and at the same time endanger – some of the most vulnerable patients in dialysis, oncology, and parenteral nutrition. Catheter-related infections not only require expensive in-hospital treatment – they also raise the mortality rate among those affected to a considerable degree. (4)
But what’s the alternative to antibiotics? To effectively prevent CRBSIs, you need a lock solution that kills all types of germs, including the dormant ones – without generating bacterial resistances. This is where taurolidine comes in:
- Taurolidine is a derivative of taurine, an amino acid which naturally occurs in the human body (and is commonly used for energy drinks). (7)
- It exerts a strong antimicrobial activity against more than 500 types of germs, including those commonly found in catheter biofilm (e.g. E. coli and S. epidermis). (8)
- When it enters the body, taurolidine quickly degrades to taurine, carbon dioxide, and water. (7)
Due to its broad antimicrobial efficacy (and lack of unwanted side effects), taurolidine forms the basis for all lock solutions from the TauroLock™ portfolio. Depending on the patient’s needs, it can be combined with different active ingredients: citrate, heparin, and/or urokinase. The main purpose of all product variations remains the same: Prevent catheter-related complications (such as the formation of biofilm) and thereby protect patients against infections.
- Austin et al. Comparative effect of seven prophylactic locks to prevent biofilm biomass and viability in intravenous catheters. J Antimicrob Chemother 2022. DOI: 10.1093/jac/dkac181
- Rittershaus et al. The Normalcy of Dormancy. Cell Host Microbe. 2013 June 12; 13(6): 643–651. DOI:10.1016/j.chom.2013.05.012
- Li et al. Mechanisms and Control Measures of Mature Biofilm Resistance to Antimicrobial Agents in the Clinical Context. ACS Omega 2020, 5, 22684−22690. DOI: 10.1021/acsomega.0c02294
- Hogan et al. In Vitro Approach for Identification of the Most Effective Agents for Antimicrobial Lock Therapy in the Treatment of Intravascular Catheter-Related Infections Caused by Staphylococcus aureus. Antimicrob Agents Chemother 2016. DOI: 10.1128/AAC.02885-15
- Labriola. Antibiotic locks for the treatment of catheter-related blood stream infection: Still more hope than data. Semin Dial 2019. DOI: 10.1111/sdi.12807
- Taleb et al. Effectiveness of salvage catheters in home parenteral nutrition: A single-center study and systematic literature review. Clin Nutr ESPEN 2023. DOI: 10.1016/j.clnesp.2023.04.026
- https://www.taurolock.com/en/about/faq
- Torres-Viera et al. Activities of Taurolidine In Vitro and in Experimental Enterococcal Endocarditis. Antimicrob Agents Chemother 2000. DOI: 10.1128/AAC.44.6.1720-1724.2000


